.
Esters in Cannabis: Unique Compounds Responsible for Weed's Fruity and Sweet Aromas
You know all about cannabinoids and you're familiar with terpenes. But have you heard of marijuana esters? This emerging class of cannabis chemicals has researchers, breeders, and consumers equally excited. Esters add unique aromas to certain strains, contributing hints of pineapple, blackberry, and apple. Learn all about them here!
Contents:
- Unveiling the world of cannabis esters
- What are esters?
- The unique chemistry of cannabis esters
- Where else are esters found in nature?
- What flavours and aromas do esters add to different weed strains?
- Do esters contribute to the entourage effect?
- Tips for preserving cannabis aromas rich in esters
- The future of esters in the cannabis industry
Key Points
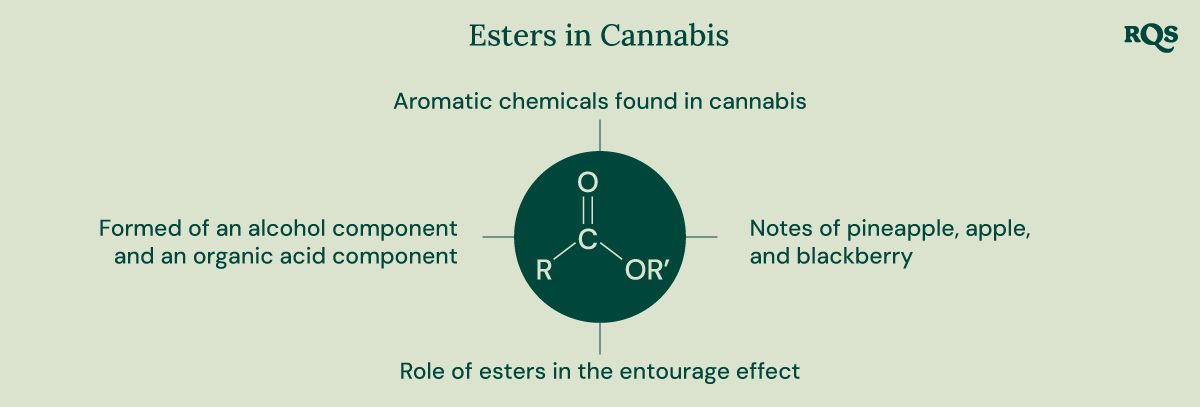
- Esters are aromatic chemicals found in cannabis and other plants.
- These chemicals are formed of an alcohol component and an organic acid component.
- Esters contribute unique flavours to certain strains, including notes of pineapple, apple, and blackberry.
- Cannabinoid esters are cannabinoid–terpene pairings fused together with an ester bond.
- Researchers are keen to identify the role of esters in the entourage effect.
When it comes to the captivating aromas of cannabis, terpenes often steal the spotlight. However, advances in weed research have unveiled that other chemicals influence the unique smells and flavours of each strain. Esters are one such group of aromatic molecules, contributing sweet and fruity notes to many cultivars.
Alongside their aromatic influence, esters are also being probed by researchers for their influence on the effects of cannabis. The research remains early, but further studies will hopefully reveal if esters have a notable impact on the entourage effect, alongside cannabinoids and terpenes.
In this comprehensive exploration of cannabis esters, we’ll dive into the science behind these aromatic molecules. By the end of this article, you’ll have a deeper understanding of yet another group of chemicals found in your favourite plant.

Unveiling the World of Cannabis Esters
Cannabis plants possess complex biological machinery in the form of genes, cells, and enzymes. Together, these components are genetically wired to produce a long list of secondary metabolites—over 500 in total. Cannabinoids and terpenes are the most abundant of these molecules, however, plants also rely on the production of many other chemicals to help them survive and thrive, including esters.
Plants invest resources into the creation of these molecules for several key purposes, including the attraction of pollinators and beneficial insects as well as protection against pathogens. While terpenes play a crucial role in the aromas of individual cannabis strains, esters produce unique tastes and scents that terpenes simply don’t. These notes are primarily sweet, reminiscent of pineapple, strawberry, and banana. However, they also contribute herbal flavours such as mint and jasmine.
What Are Esters?
Esters are organic compounds that play a key role in the aromas of many plant species. They’re formed through the process of esterification, a reaction that occurs between an alcohol and a carboxylic acid molecule.
Esters are widespread throughout nature—and not just in plants. While they occur as important components in fruits, flowers, and herbs, they’re also found in animal fats. As secondary metabolites, they contribute to survival and offer other specialised functions. Explore their roles below.
-
Ecological Advantages
Esters provide plants with distinct ecological advantages that help them to thrive. Some of them work as toxic compounds that discourage pests, larger herbivores, and pathogens from feeding on leaves, stems, and other anatomical components. Other esters do the opposite, attracting pollinators with attractive scents.
-
Stress Tolerance
Plants also produce esters to help them deal with abiotic (non-living) sources of stress. Some of these compounds are useful in helping plants protect themselves against harsh UV radiation, whereas surface wax esters accumulate during drought conditions and improve tolerance to extreme temperatures and reduced access to water. Some esters also modify plant cell walls during drought, contributing to fortification.
-
Stomata Function
Plant leaves are covered in tiny pores that open and close to release and draw in gases, such as carbon dioxide, oxygen, and water. Some plant esters, such as methyl jasmonate, contribute to stomatal closure to assist gas exchange and to help defend against pests looking to gain entry.
The Unique Chemistry of Cannabis Esters
The esters that occur in cannabis plants fall into two distinct groups: non-cannabinoid esters and cannabinoid esters. Non-cannabinoid esters feature an alcohol component and an organic acid component connected by an ester linkage.
Examples of non-cannabinoid esters found in the marijuana plant include ethyl hexanoate and n-propyl hexanoate. These chemicals generally have a molecular weight of 100–300 g/mol. Because they typically have a lower boiling point than their parent alcohols and acids, they’re volatile compounds and therefore play an important role in plant aroma.
Cannabis and other plants produce esters through specific biochemical pathways. Starting out with the basic precursor molecules, enzymes such as esterases and acyltransferases catalyse reactions that create more complex ester molecules.
However, several factors can cause esters to change and degrade. Hydrolysis, a reaction in which a water molecule breaks chemical bonds, can cause esters to revert back to their more simple alcohol and acid parent molecules. Likewise, oxidation can degrade esters, and their relative thermal instability means high temperatures can cause them to break down.
-
A Look at Cannabinoid Esters
Cannabinoid esters are fascinating molecules. They’re essentially modified cannabinoid compounds often produced via the fusion of terpenes through the esterification process. Typically, an ester linkage binds a terpene or another organic molecule onto a cannabinoid core, such as THC, CBD, or CBG.
Examples of cannabinoid esters include β-fenchyl Δ9-tetrahydrocannabinolate, α-terpenyl Δ9-tetrahydrocannabinolate, and THC-O-acetate. Because they’re composed of heavier parent molecules, they feature a heavier molecular mass. For example, THC-o-acetate clocks in at around 350 g/mol. Cannabinoid esters, much like cannabinoids themselves, are highly lipophilic. This means they readily dissolve into fats and oils and feature poor solubility in water.
Where Else Are Esters Found in Nature?
Esters are found in abundance throughout nature, including in various anatomical components of many plants. For example, the ester ethyl-2-methylbutyrate gives apples their sweet and fruity aroma. Likewise, both ethyl butyrate and ethyl hexanoate contribute to the unique tastes and flavours of pineapple.
Esters are also pivotal in the scents of various flowers. The ester geranyl acetate underpins the smell of roses, whereas benzyl acetate and other esters are among the key aromatic components of jasmine flowers.
The waxy nature of esters also makes them an important constituent in many animal waxes and oils, including beeswax, sperm whale oil, and sheep wool fat. Esters are also used as pheromones in some insect species, including ants. Many marine organisms also produce esters. Algae, corals, and sponges all produce esters for various purposes, including as defence compounds.
What Flavours and Aromas Do Esters Add to Different Weed Strains?
The research surrounding esters in cannabis remains early, especially compared to investigations into cannabinoids and terpenes. However, scientists have begun to explore how specific compounds within this chemical group impact the aroma of some weed strains. Check out some of the primary esters found in weed below.
-
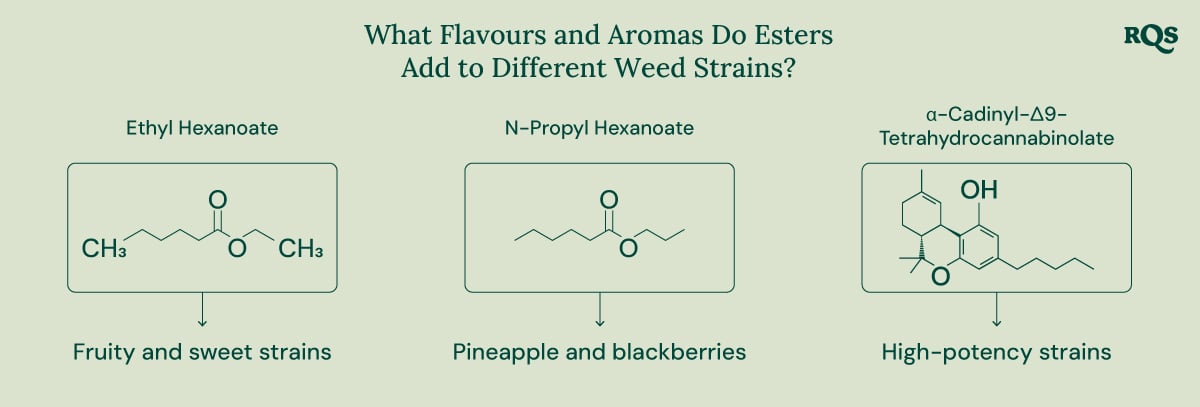
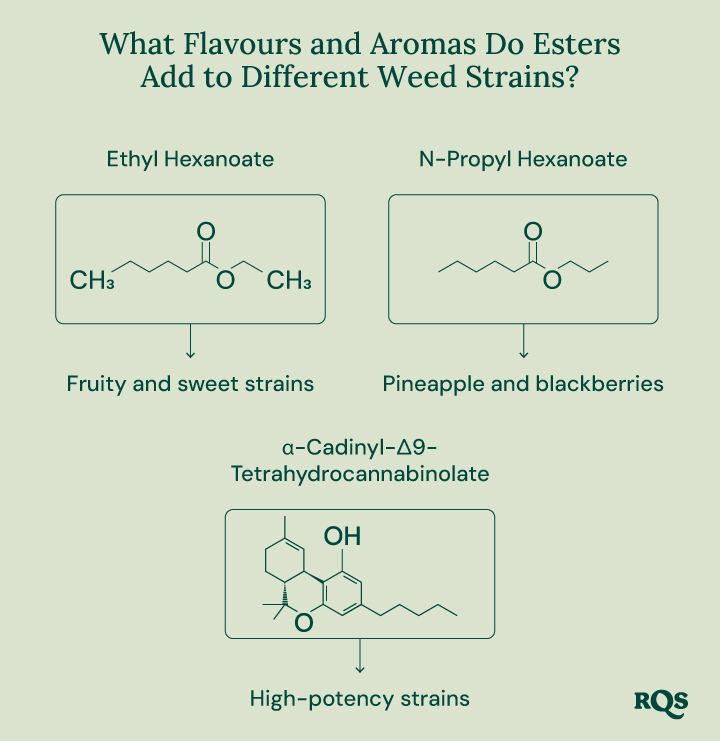
Ethyl Hexanoate
Ethyl hexanoate features a low odour threshold, meaning only small concentrations are required for the molecule to have a big impact on cannabis aroma profiles. This compound consists of a hexanoic acid backbone esterified with an ethyl group. It has a linear structure and the chemical formula C₈H₁₆O₂. With a molecular weight of around 144 g/mol, ethyl hexanoate contributes a fruity, sweet, apple-like aroma to certain cannabis strains.
-
N-Propyl Hexanoate
N-Propyl hexanoate also plays an important role in the quintessential weed smell. With a chemical formula of C₉H₁₈O₂, this ester consists of a hexanoic acid backbone bound to a propyl group. This phytochemical underpins the fruity scents found in many cannabis varieties. However, it also adds unique notes of pineapple and blackberries.
-
α-Cadinyl-Δ9-Tetrahydrocannabinolate
Researchers have also started to unveil the unique cannabinoid esters[1] produced in marijuana plants. Despite its chemical name, this ester has a fairly simple chemical makeup. It’s associated with high-potency cannabis varieties and results from the combination of the cannabinoid acid THCA and the terpene epi-borneol linked through enzymatic esterification.
α-Cadinyl-Δ9-tetrahydrocannabinolate possesses a relatively heavy molecular weight of around 482 g/mol. Due to the presence of epi-borneol in its structure, this phytochemical likely adds scents of herbs and wood to select strains.
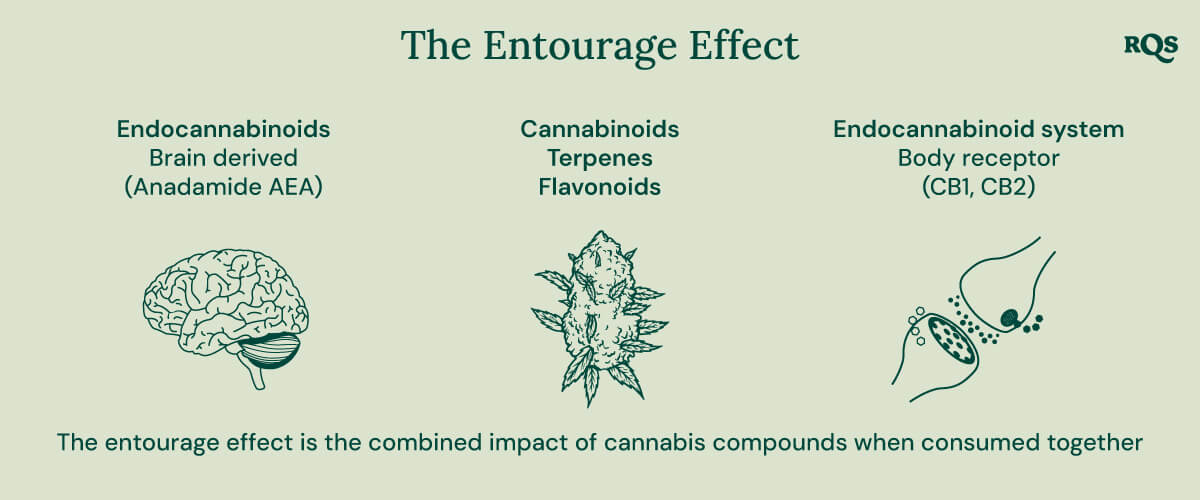
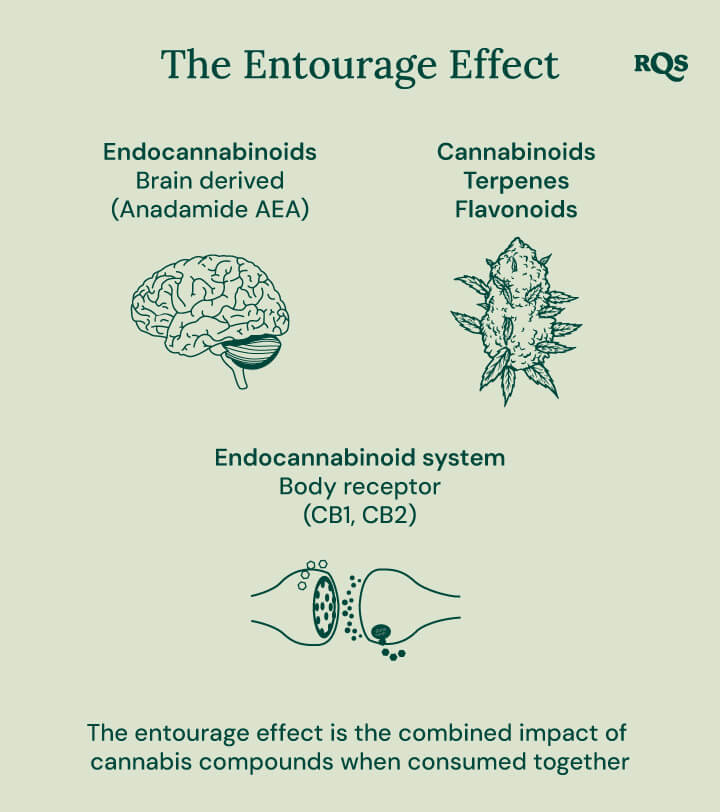
Do Esters Contribute to the Entourage Effect?
The entourage effect describes the synergistic action between chemical components found in cannabis. Cannabinoids and terpenes, when administered in combination, are thought to induce novel physiological effects. For example, some cannabinoid–terpene combinations result in the amplification of certain compounds’ effects and the dampening of others.
No studies have specifically examined the effects of esters on the endocannabinoid system. However, many terpenes and cannabinoids bind to the receptors of this network. If esters are also found to directly or indirectly interface with this system, these compounds could play a major role in future extract formulations and breeding operations.
Tips for Preserving Cannabis Aromas Rich in Esters
Esters contribute significant notes to the overall aroma of cannabis. However, much like terpenes, they’re prone to degrading when stored improperly. Check out the tips below to keep your buds fresh and these flavourful molecules intact:
- Store in a cool, dark place: Heat can degrade esters. Keep your stash in a cool, dark place to preserve those special flavours and aromas.
- Control humidity: Use humidity control packs to maintain optimal humidity in your stash jars. Excess moisture can create a hay-like taste and increase the chances of mould.
- Minimise air exposure: Use airtight storage containers to reduce the risk of esters oxidising.
- Vape at low temperatures: Esters have low boiling points. To experience the flavours to their fullest, use low temperature settings when vaping.
The Future of Esters in the Cannabis Industry
Esters are just beginning to emerge as molecules of interest in the cannabis industry. Researchers have only identified a handful of them so far, but already their big contribution to the aroma and flavour of cannabis strains has scientists, breeders, and consumers excited.
Future studies will hopefully identify more of these compounds, pinpoint their unique scents, and unveil their possible synergistic action and influence on the effects of particular strains. Until then, every time you detect flavours of fruit, pineapple, apple, and blackberry, know that you have esters—at least in part—to thank!
- Cannabinoid Ester Constituents from High-Potency Cannabis sativa - PMC https://pmc.ncbi.nlm.nih.gov









































